Post-acute and Chronic Kidney Function Outcomes of COVID-19 in Children and Adolescents: An EHR Cohort Study from the RECOVER Initiative
Lu Li, Ting Zhou, Yiwen Lu, Jiajie Chen, Yuqing Lei, Qiong Wu, Jonathan Arnold, Michael J. Becich, Yuriy Bisyuk, Saul Blecker, and 26 more authors
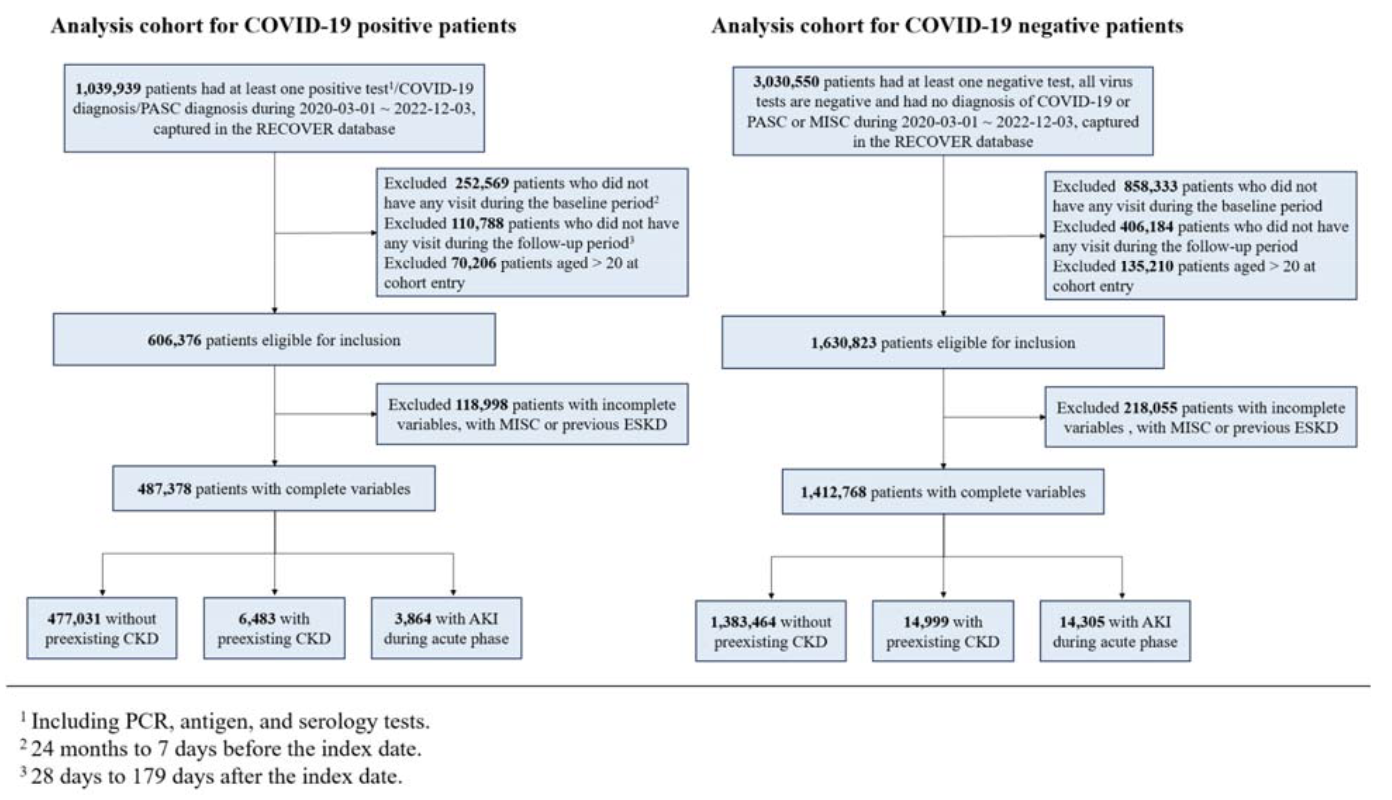
We investigated the risks of post-acute and chronic adverse kidney outcomes of SARS-CoV-2 infection in the pediatric population via a retrospective cohort study using data from the RECOVER program. We included 1,864,637 children and adolescents under 21 from 19 children’s hospitals and health institutions in the US with at least six months of follow-up time between March 2020 and May 2023. We divided the patients into three strata: patients with pre-existing chronic kidney disease (CKD), patients with acute kidney injury (AKI) during the acute phase (within 28 days) of SARS-CoV-2 infection, and patients without pre-existing CKD or AKI. We defined a set of adverse kidney outcomes for each stratum and examined the outcomes within the post-acute and chronic phases after SARS-CoV-2 infection. In each stratum, compared with the non-infected group, patients with COVID-19 had a higher risk of adverse kidney outcomes. For patients without pre-existing CKD, there were increased risks of CKD stage 2+ (HR 1.20; 95% CI: 1.13-1.28) and CKD stage 3+ (HR 1.35; 95% CI: 1.15-1.59) during the post-acute phase (28 days to 365 days) after SARS-CoV-2 infection. Within the post-acute phase of SARS-CoV-2 infection, children and adolescents with pre-existing CKD and those who experienced AKI were at increased risk of progression to a composite outcome defined by at least 50% decline in estimated glomerular filtration rate (eGFR), eGFR <15 mL/min/1.73m2, End Stage Kidney Disease diagnosis, dialysis, or transplant.Lay abstract This study examined the impact of COVID-19 on kidney health in children and adolescents under 21 years old in the United States. Using data from the RECOVER program, we analyzed the health records of 1,864,637 young individuals from 19 hospitals and health institutions between March 2020 and May 2023. The study focused on three groups: those with pre-existing chronic kidney disease (CKD), those who experienced acute kidney injury (AKI) during the initial COVID-19 infection, and those without any prior kidney issues. The results showed that children and adolescents who had COVID-19 were at a higher risk of developing serious kidney problems later on, even if they had no previous kidney conditions. This research highlights the long-term effects of COVID-19 on kidney health in young people and underscores the importance of monitoring kidney function in pediatric COVID-19 patients.Competing Interest StatementThe authors have declared no competing interest.Funding StatementThis study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). For more information on RECOVER, visit https://recovercovid.org/Author DeclarationsI confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.YesThe details of the IRB/oversight body that provided approval or exemption for the research described are given below:Ethics committee/IRB of University of Pennsylvania gave ethical approval for this workI confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.YesI understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).YesI have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.YesThe data is available through applying through the RECOVER program.

 Post-acute and Chronic Kidney Function Outcomes of COVID-19 in Children and Adolescents: An EHR Cohort Study from the RECOVER InitiativemedRxiv, 2024
Post-acute and Chronic Kidney Function Outcomes of COVID-19 in Children and Adolescents: An EHR Cohort Study from the RECOVER InitiativemedRxiv, 2024 Minimal Cycle Representatives in Persistent Homology Using Linear Programming: An Empirical Study With User’s GuideFrontiers in Artificial Intelligence, 2021
Minimal Cycle Representatives in Persistent Homology Using Linear Programming: An Empirical Study With User’s GuideFrontiers in Artificial Intelligence, 2021